Unified total synthesis of the brevianamide alkaloids enabled by chemical investigations into their biosynthesis†
Abstract
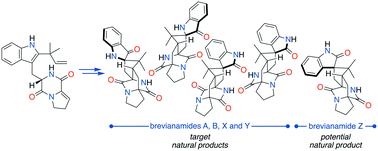
The bicyclo[2.2.2]diazaoctane alkaloids are a vast group of natural products which have been the focus of attention from the scientific community for several decades. This interest stems from their broad range of biological activities, their diverse biosynthetic origins, and their topologically complex structures, which combined make them enticing targets for chemical synthesis. In this article, full details of our synthetic studies into the chemical feasibility of a proposed network of biosynthetic pathways towards the brevianamide family of bicyclo[2.2.2]diazaoctane alkaloids are disclosed. Insights into issues of reactivity and selectivity in the biosynthesis of these structures have aided the development of a unified biomimetic synthetic strategy, which has resulted in the total synthesis of all known bicyclo[2.2.2]diazaoctane brevianamides and the anticipation of an as-yet-undiscovered congener.


 Please wait while we load your content...
Please wait while we load your content...