Supramolecular polymerization of electronically complementary linear motifs: anti-cooperativity by attenuated growth†
Abstract
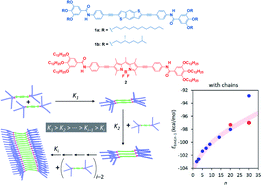
Anti-cooperative supramolecular polymerization by attenuated growth exhibited by self-assembling units of two electron-donor benzo[1,2-b:4,5-b′]dithiophene (BDT) derivatives (compounds 1a and 1b) and the electron-acceptor 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) (compound 2) is reported. Despite the apparent cooperative mechanism of 1 and 2, AFM imaging and SAXS measurements reveal the formation of small aggregates that suggest the operation of an anti-cooperative mechanism strongly conditioned by an attenuated growth. In this mechanism, the formation of the nuclei is favoured over the subsequent addition of monomeric units to the aggregate, which finally results in short aggregates. Theoretical calculations show that both the BDT and BODIPY motifs, after forming the initial dimeric nuclei, experience a strong distortion of the central aromatic backbone upon growth, which makes the addition of successive monomeric units unfavourable and impedes the formation of long fibrillar structures. Despite the anti-cooperativity observed in the supramolecular polymerization of 1 and 2, the combination of both self-assembling units results in the formation of small co-assembled aggregates with a similar supramolecular polymerization behaviour to that observed for the separate components.


 Please wait while we load your content...
Please wait while we load your content...