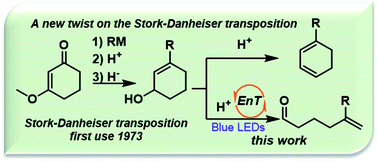
A new twist for Stork-Danheiser products enabled by visible light mediated trans-cyclohexene formation; access to acyclic distal enones†
Abstract
Herein, we investigate the use of visible light to indirectly drive ring opening in unstrained 6- and 7-membered ring systems via reaction with a transiently generated trans-cycloalkene. Identification of conditions that capture visible light energy in the form of ring strain was key to success. Under mildly acidic conditions, cycloalkenols were shown to undergo formally endothermic ring-opening isomerization to give acyclic exo-methylene and distal ketones or aldehydes in high yields. Ultimately, this work demonstrates the ability of cycloalkenes to capture visible light energy and its use to drive both kinetically and thermally unfavorable rearrangements.


 Please wait while we load your content...
Please wait while we load your content...