Degradation mechanism of a lignin model compound during alkaline aerobic oxidation: formation of the vanillin precursor from the β-O-4 middle unit of softwood lignin†
Abstract
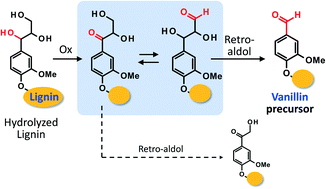
Vanillin (4-hydroxy-3-methoxybenzaldehyde), one of the platform chemicals in industry, has been industrially obtained by alkaline aerobic oxidation of softwood lignin, a major component of lignocellulosics. A major reaction pathway of vanillin formation from the lignin polymer involves oxidation of an end group carrying a glycerol moiety produced by alkaline hydrolysis of a β-O-4 linkage in a middle unit of the lignin polymer to a C1 aldehyde moiety. This study presents the oxidation of veratryl glycerol [threo-1-(3,4-dimethoxyphenyl)propane-1,2,3-triol, VGL] as a model compound of the end group with the glycerol moiety at 120 °C under air in NaOH aq., aiming for the elucidation of detailed mechanisms of the oxidation of the C3 side-chain of VGL. Our experimental observations were explained by the assumption that the initial stage of the aldehyde formation was oxidation of the C3 side-chain of VGL at the α-position to a Cα![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moiety, followed by isomerization of this intermediate to a Cγ
O moiety, followed by isomerization of this intermediate to a Cγ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O compound, which finally underwent a retro-aldol reaction to give the α-aldehyde vanillin precursor. This consideration was supported also by our computational investigation at the SCS-MP2//DFT(M06-2X) level of theory on the retro-aldol reactions.
O compound, which finally underwent a retro-aldol reaction to give the α-aldehyde vanillin precursor. This consideration was supported also by our computational investigation at the SCS-MP2//DFT(M06-2X) level of theory on the retro-aldol reactions.


 Please wait while we load your content...
Please wait while we load your content...