A terpene cyclase from Aspergillus ustus is involved in the biosynthesis of geosmin precursor germacradienol†
Abstract
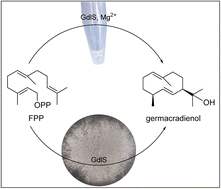
The earthy odor of geosmin with a C12 skeleton is known from bacteria, fungi and plants. The sesquiterpenoid germacradien-11-ol (germacradienol) is a crucial intermediate in the biosynthesis of geosmin. A bifunctional terpene cyclase for germacradienol formation and its degradation to geosmin had been described in bacteria. Terpene cyclases were also suggested for geosmin formation in basidiomycetes, but not reported for ascomycetes. We identified a putative terpene cyclase in Aspergillus ustus with low sequence homology to N-termini of the bacterial germacradienol/geosmin synthases. Heterologous expression in Aspergillus nidulans and biochemical characterization led to the identification of the geosmin precursor germacradienol as the sole detected enzyme product. Germacradienol synthase (GdlS) uses strictly farnesyl diphosphate as substrate for cyclization and requires Mg2+ for its reaction. Multiple sequence alignments with known enzymes indicate the presence of the highly conserved catalytic residues including the DDXXD motif for Mg2+ binding. Phylogenetic analysis suggests different clades of bacterial germacradienol/geosmin synthases and terpene cyclases from fungi.


 Please wait while we load your content...
Please wait while we load your content...