Dearomative gem-diprenylation of hydroxynaphthalenes by an engineered fungal prenyltransferase†
Abstract
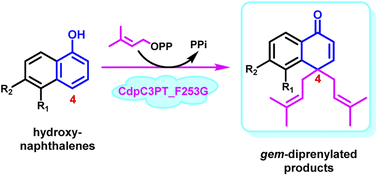
Prenylation usually improves structural diversity and bioactivity in natural products. Unlike the discovered enzymatic gem-diprenylation of mono- and tri-cyclic aromatic systems, the enzymatic approach for gem-diprenylation of bi-cyclic hydroxynaphthalenes is new to science. Here we report an enzymatic example for dearomative C4 gem-diprenylation of α-hydroxynaphthalenes, by the F253G mutant of a fungal prenyltransferase CdpC3PT. Experimental evidence suggests a sequential electrophilic substitution mechanism. We also explained the alteration of catalytic properties on CdpC3PT after mutation on F253 by modeling. This study provides a valuable addition to the synthetic toolkit for compound prenylation and it also contributes to the mechanistic study of prenylating enzymes.


 Please wait while we load your content...
Please wait while we load your content...