Design and synthesis of novel 3-triazolyl-1-thiogalactosides as galectin-1, -3 and -8 inhibitors†
Abstract
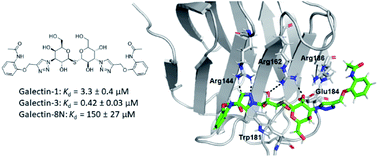
Galectins are galactoside-binding proteins that play a role in various pathophysiological conditions, making them attractive targets in drug discovery. We have designed and synthesised a focused library of aromatic 3-triazolyl-1-thiogalactosides targeting their core site for binding of galactose and a subsite on its non-reducing side. Evaluation of their binding affinities for galectin-1, -3, and -8N identified acetamide-based compound 36 as a suitable compound for further affinity enhancement by adding groups at the reducing side of the galactose. Synthesis of its dichlorothiophenyl analogue 59 and the thiodigalactoside analogue 62 yielded promising pan-galectin inhibitors.


 Please wait while we load your content...
Please wait while we load your content...