Highly active postspinel-structured catalysts for oxygen evolution reaction†
Abstract
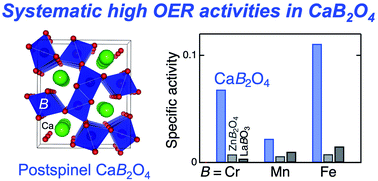
The rational design principle of highly active catalysts for the oxygen evolution reaction (OER) is desired because of its versatility for energy-conversion applications. Postspinel-structured oxides, CaB2O4 (B = Cr3+, Mn3+, and Fe3+), have exhibited higher OER activities than nominally isoelectronic conventional counterparts of perovskite oxides LaBO3 and spinel oxides ZnB2O4. Electrochemical impedance spectroscopy reveals that the higher OER activities for CaB2O4 series are attributed to the lower charge-transfer resistances. A density-functional-theory calculation proposes a novel mechanism associated with lattice oxygen pairing with adsorbed oxygen, demonstrating the lowest theoretical OER overpotential than other mechanisms examined in this study. This finding proposes a structure-driven design of electrocatalysts associated with a novel OER mechanism.


 Please wait while we load your content...
Please wait while we load your content...