Aliphatic sulfonyl fluoride synthesis via reductive decarboxylative fluorosulfonylation of aliphatic carboxylic acid NHPI esters†
Abstract
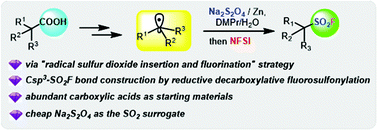
Based on a radical sulfur dioxide insertion and fluorination strategy, we have developed an efficient method for aliphatic sulfonyl fluoride synthesis using abundant carboxylic acids, a reductant, a sulfur dioxide surrogate and the electrophilic fluorination reagent N-fluorobenzenesulfonimide (NFSI) under reduction conditions. This protocol provides a convenient synthetic pathway for various aliphatic sulfonyl fluorides and tolerates a wide range of functional groups.
- This article is part of the themed collection: FOCUS: Recent progress on click chemistry and bioorthogonal chemistry


 Please wait while we load your content...
Please wait while we load your content...