Ni(ii)-Catalyzed intermolecular selective Heck-type arylation of unactivated alkenes with arylboronic acids†
Abstract
The Ni(II)-catalyzed intermolecular arylation of unactivated alkenes with arylboronic acids has been disclosed for the first time. This alkene arylation exhibits excellent E/Z selectivity and regioselectivity (γ-selectivity) to provide the corresponding γ-aryl substituted β,γ-unsaturated carboxylic acid derivatives with E-selectivity. A Heck-type mechanism is proposed, wherein the 8-aminoquinoline auxiliary group takes the crucial role of the alkenes in the insertion/β-H elimination steps.
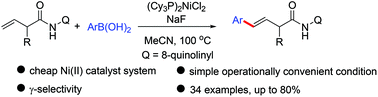


 Please wait while we load your content...
Please wait while we load your content...