Carbon cage isomers and magnetic Dy⋯Dy interactions in Dy2O@C88 and Dy2C2@C88 metallofullerenes†
Abstract
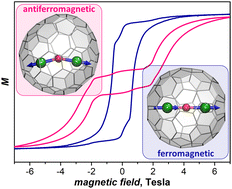
Three isomers of Dy2O@C88 and two isomers of Dy2C2@C88 were synthesized and structurally characterized by single-crystal X-ray diffraction, vibrational spectroscopy, and DFT calculations. Both types of clusterfullerenes feature 4-fold electron transfer to the carbon cage, thus resulting in the same carbon cage isomers identified as C1(26), Cs(32), and D2(35). The studies of Dy⋯Dy superexchange interactions in Dy2O and Dy2C2 clusters revealed that the O2− bridge favors antiferromagnetic coupling whereas the acetylide group C22− supports ferromagnetic coupling of Dy magnetic moments. The strength of the coupling showed a considerable variability in different cage isomers. All metallofullerenes exhibited slow relaxation of magnetization and magnetic hysteresis. In Dy2O@C88 isomers the hysteresis remained open up to 7–9 K, while in Dy2C2@C88 the hysteresis loops were closed already at 2.5 K. This study demonstrated that both the endohedral bridge between metal atoms and the fullerene cage play an important role in magnetic interactions and relaxation of magnetization.
- This article is part of the themed collection: FOCUS: Metal and Metal-Containing Clusters


 Please wait while we load your content...
Please wait while we load your content...