Heterovalent chalcogen bonding: supramolecular assembly driven by the occurrence of a tellurium(ii)⋯Ch(i) (Ch = S, Se, Te) linkage†
Abstract
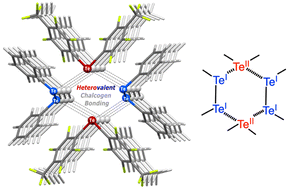
The dichalcogenides Ph2Ch2 (Ch = S, Se, Te) were cocrystallized with perfluorinated chalcogen bond donors TolF2Te and PyF2Te (TolF = 4-CF3C6F4, PyF = 4-NC5F4) to obtain the 1 : 1 cocrystals TolF2Te·Ph2Ch2 (Ch = S 1, Se 2, Te 3) and PyF2Te·Ph2Se2 (4). In the X-ray structures of 1–4, heterovalent TeII⋯ChI (Ch = S, Se, Te) chalcogen bonding was identified on consideration of the geometrical parameters and, in addition, based on the results of appropriate density functional theory (DFT) calculations including quantum theory of atoms-in-molecules (QTAIM), noncovalent interaction plot (NCIplot) analysis, molecular electrostatic potential surfaces (MEP), and atoms-in-molecules (AIM) charge analysis. The binding energy in the dimeric structure is in the range between −9.7 and −12.9 kcal mol−1, where the contribution of the heterovalent chalcogen bonding ranges from −4.7 to −6.5 kcal mol−1. In the TeII⋯ChI moiety, the TeII center plays the role of an electrophilic partner, while the chalcogens in the lower oxidation state, 1+, exhibit nucleophilic properties. The heterovalent TeII⋯ChI (Ch = Se, Te) chalcogen bonding was thus used for the targeted noncovalent integration of two Ch centers in different oxidation states.


 Please wait while we load your content...
Please wait while we load your content...