Ligand-regulated metal–organic frameworks for synergistic photoredox and nickel catalysis†
Abstract
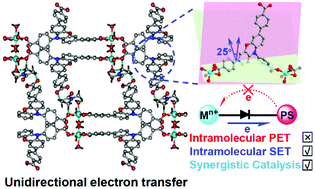
Synergistic photoredox and nickel catalytic cross-coupling systems have attracted great attention as a promising methodology for the production of aryl C–N bonds under mild conditions. They are also an extreme challenge because they are dramatically affected by the extremely widespread photoinduced electron transfer (PET) from the organic photoredox catalysts to the NiII. By regulating the geometry configuration of ligands to compulsively generate the twisting of the conjugation between the dye-based ligands and the NiII-carboxylate nodes in the metal–organic frameworks (MOFs), herein, we report a new heterogeneous approach to directly overcome this inherent challenge. The rigid plane structure of phenoxazine as compared to triphenylamine promotes the twisted NiII-phenoxazine conjunction in the metal–organic frameworks and guarantees the diode-like photoelectron flow characteristics, which successfully execute the reversed-directional ground-state electronic conductivity, where the unwanted photoinduced electron transfer processes from the phenoxazine-moieties to the NiII sites are potentially avoided. This synergistic photoredox and nickel catalytic approach, which offers control over the direction of the electron transfer, provides a mild concise C–N cross-coupling strategy utilizing a NiII catalyst along with a photocatalyst that operates at room temperature under visible light and is extendable to aryl bromide and aryl chloride systems.


 Please wait while we load your content...
Please wait while we load your content...