New mechanism for autoxidation of polyolefins: kinetic Monte Carlo modelling of the role of short-chain branches, molecular oxygen and unsaturated moieties†
Abstract
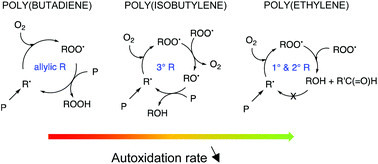
In this work, a bivariate kinetic Monte Carlo (kMC) model is constructed to study autoxidation, which is the degradation of polymers in the presence of oxygen. The use of computational methods for the determination of rate coefficients as input for the model is illustrated. Focus is on the presence of short-chain branches (SCB) and unsaturated moieties and their role in the fate of alkyl, alkoxyl and alkylperoxyl radicals in the autoxidation mechanism. The autoxidation kinetics are studied for three model polymers, namely poly(ethylene) (reference case), poly(butadiene) (presence of allylic hydrogens), and poly(isobutylene) (presence of quaternary carbon atoms). Using the kMC model, reaction path analysis shows that the autoxidation mechanism for each of the polymer types follows a chain reaction mechanism, but that the presence of branches/unsaturated moieties influences the dominant reaction pathway in the autoxidation mechanism, and thus also the autoxidation rate. It is shown that the influence of varying oxygen concentration and initiation rate coefficient (e.g. to simulate variable ultraviolet (UV) light intensity) on the dominant pathway is small as their role is mainly situated in the first steps of the chain mechanism.


 Please wait while we load your content...
Please wait while we load your content...