BiCl3 catalyzed synthesis of 2-amino-1,4-naphthoquinones and 1,4-naphthoquinon-2-sulfides and one-pot sequential amine-arylation of 1,4-naphthoquinone†
Abstract
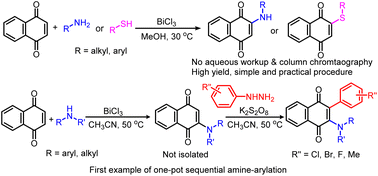
Using the Lewis acid catalyst BiCl3, a new method for the synthesis of 2-amino-1,4-naphthoquinones and 1,4-naphthoquinon-2-sulfides from 1,4-naphthoquinone has been devised. This method has a broad substrate scope and product extraction is easy. It is low-cost, requires mild and sustainable reaction conditions and results in superior product yields. Additionally, this study presents the first technique for one-pot sequential amine-arylation with amines and arylhydrazines/K2S2O8 to produce arylated 2-amino-1,4-naphthoquinones directly from 1,4-naphthoquinone. This operationally straightforward generation of aryl radicals from arylhydrazines that undergo a free radical coupling reaction is well demonstrated by radical trapping control experiments.


 Please wait while we load your content...
Please wait while we load your content...