Synthesis of fluorinated carbocyclic pyrimidine nucleoside analogues†
Abstract
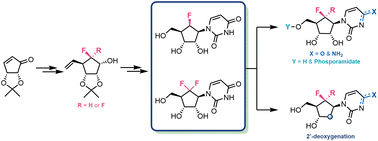
Analogues of the canonical nucleosides have a longstanding presence and proven capability within medicinal chemistry and drug discovery research. The synthesis reported herein successfully replaces furanose oxygen with CF2 and CHF in pyrimidine nucleosides, granting access to an alternative pharmacophore space. Key diastereoselective conjugate addition and fluorination methodologies are developed from chiral pool materials, establishing a robust gram-scale synthesis of 6′-(R)-monofluoro- and 6′-gem-difluorouridines. Vital intermediate stereochemistries are confirmed using X-ray crystallography and NMR analysis, providing an indicative conformational preference for these fluorinated carbanucleosides. Utilising these 6′-fluorocarbauridine scaffolds enables synthesis of related cytidine, ProTide and 2′-deoxy analogues alongside a preliminary exploration of their biological capabilities in cancer cell viability assays. This synthetic blueprint offers potential to explore fluorocarbanucleoside scaffolds, indicatively towards triphosphate analogues and as building blocks for oligonucleotide synthesis.


 Please wait while we load your content...
Please wait while we load your content...