Investigation of iron release from the N- and C-lobes of human serum transferrin by quantum chemical calculations†
Abstract
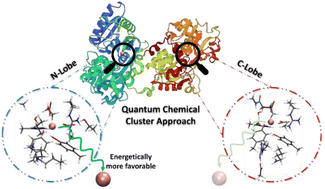
Human serum transferrin binds ferric ions with high affinity and delivers them into cells via receptor-mediated endocytosis upon a decrease in pH in the endosome. Protonation events and conformational changes are known to play an important role in iron-release though the release is not yet fully understood. Human serum transferrin consists of two similar lobes which release iron at different rates. In this study, we investigate the iron binding sites of N- and C-lobes using quantum mechanical tools, particularly, the quantum chemical cluster approach. This study supports the inevitable role of axial tyrosine for the release of iron in quantum chemical models and provides valuable information about the proton transfer pathways for the protonation of Tyr188 and Tyr517 in N- and C-lobes, respectively. The calculations show that the release process is similar in both lobes; however, the energetic differences of the release process in N- and C-lobes, demonstrated for the first time, indicated that the release of iron in the N-lobe is thermodynamically favorable, in contrast to the one in the C-lobe.


 Please wait while we load your content...
Please wait while we load your content...