Divergent cyclodimerizations of styrylnaphthols under aerobic visible-light irradiation and Brønsted acid catalysis†
Abstract
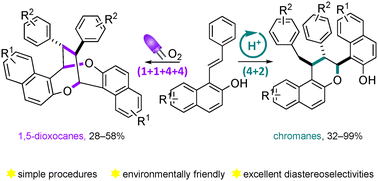
Dimeric cyclization reactions show great potential to rapidly form highly substituted complex cyclic molecules from simple starting materials. However, such an appealing process is often hampered by the lack of selectivity. Herein we report two divergent cyclodimerization reactions of 1-styrylnaphthalen-2-ol derivatives under simple and very mild reaction conditions. A stereoselective visible light-induced oxidative (1 + 1 + 4 + 4) homodimerization gave rise to highly substituted 1,5-dioxocanes in moderate yields. This transformation harnessed singlet oxygen as a safe and mild oxidant under photocatalyst-free reaction conditions. Additionally, we demonstrated that the same substrates undergo a (4 + 2) heterodimerization under Brønsted-acid catalysis to produce chromane derivatives featuring 3 contiguous tertiary stereocenters in good to high yields with excellent diastereoselectivities.
- This article is part of the themed collection: Celebrating the 20th anniversary of Organic & Biomolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...