ArPNO-catalyzed acylative kinetic resolution of tertiary alcohols: access to 3-hydroxy-3-substituted oxindoles†
Abstract
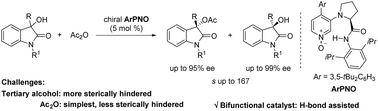
Bifunctional chiral 4-aryl-pyridine-N-oxides (ArPNO) were reported for the acylative kinetic resolution of 3-hydroxy-3-substituted oxindoles, where the oxygen acts as the nucleophilic site. Using less sterically hindered acetic anhydride, both the recovered tertiary heterocyclic alcohols and the ester products exhibited good to excellent results with s-factors up to 167. Control experiments supported the dual activation manner, where the N-oxide group and N–H proton in ArPNO were crucial for high selectivity and enhanced catalytic reactivity. Compared with the extensively used chiral NHC, isochalcogenourea, and DMAP catalysts, we found that chiral ArPNO were also efficient organocatalysts in the kinetic resolution of tertiary alcohols.


 Please wait while we load your content...
Please wait while we load your content...