Ligand-free copper-catalyzed borylative defluorination: access to gem-difluoroallyl boronic acid derivatives†
Abstract
We report a ligand-free copper-catalyzed β-borylation, defluorination of β-substituted, α-trifluoromethyl-α,β-unsaturated esters. The reaction affords geminal-difluoroallyl boronic acid derivatives in moderate to good yield. The reaction was tolerant of various substrates, and the utility of products was demonstrated in the defluorinative functionalization of the difluoroalkene to afford enol ethers.
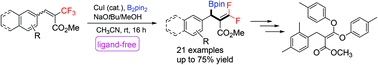
- This article is part of the themed collection: p-Block Lewis Acids in Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...
