Protein encapsulation within the internal cavity of a bacterioferritin†
Abstract
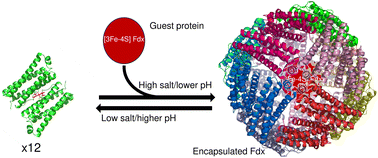
The thermal and chemical stability of 24mer ferritins has led to attempts to exploit their naturally occurring nanoscale (8 nm) internal cavities for biotechnological applications. An area of increasing interest is the encapsulation of molecules either for medical or biocatalysis applications. Encapsulation requires ferritin dissociation, typically induced using high temperature or acidic conditions (pH ≥ 2), which generally precludes the inclusion of fragile cargo such as proteins or peptide fragments. Here we demonstrate that minimizing salt concentration combined with adjusting the pH to ≤8.5 (i.e. low proton/metal ion concentration) reversibly shifts the naturally occurring equilibrium between dimeric and 24meric assemblies of Escherichia coli bacterioferritin (Bfr) in favour of the disassembled form. Interconversion between the different oligomeric forms of Bfr is sufficiently slow under these conditions to allow the use of size exclusion chromatography to obtain wild type protein in the purely dimeric and 24meric forms. This control over association state was exploited to bind heme at natural sites that are not accessible in the assembled protein. The potential for biotechnological applications was demonstrated by the encapsulation of a small, acidic [3Fe-4S] cluster-containing ferredoxin within the Bfr internal cavity. The capture of ∼4–6 negatively charged ferredoxin molecules per cage indicates that charge complementarity with the inner protein surface is not an essential determinant of successful encapsulation.


 Please wait while we load your content...
Please wait while we load your content...