White light emission from lead-free mixed-cation doped Cs2SnCl6 nanocrystals†
Abstract
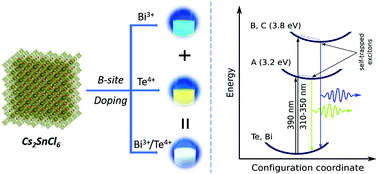
We have designed a synthesis procedure to obtain Cs2SnCl6 nanocrystals (NCs) doped with metal ion(s) to emit visible light. Cs2SnCl6 NCs doped with Bi3+, Te4+ and Sb3+ ions emitted blue, yellow and red light, respectively. In addition, NCs simultaneously doped with Bi3+ and Te4+ ions were synthesized in a single run. Combination of both dopant ions together gives rise to the white emission. The photoluminescence quantum yields of the blue, yellow and white emissions are up to 26.5, 28, and 16.6%, respectively under excitation at 350, 390, and 370 nm. Pure white-light emission with CIE chromaticity coordinates of (0.32, 0.33) and (0.32, 0.32) at 340 and 370 nm excitation wavelength, respectively, was obtained. The as-prepared NCs were found to demonstrate a long-time stability, resistance to humidity, and an ability to be well-dispersed in polar solvents without property degradation due to their hydrophilicity, which could be of significant interest for wide application purposes.


 Please wait while we load your content...
Please wait while we load your content...