Regioselective transformation of 3-phosphoryl benzyne intermediates to diverse phosphorus-substituted arenes†
Abstract
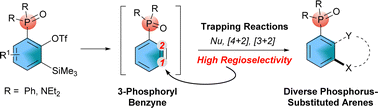
Herein, pre-functionalized benzyne precursors 5, 6 and 10 bearing a phosphoryl group were efficiently synthesized through a phospho-Fries rearrangement reaction on gram scales. Based on the versatile benzyne reactions ranging from nucleophilic addition and [3+2] and [4+2] cycloadditions to insertion reactions, a series of arylated organophosphorus molecules were readily achieved under mild reaction conditions. Attributed to the electron-withdrawing ability and the steric hindrance environment of phosphoryl groups, the reaction proceeded with high regioselectivity at the C1 position of 3-phosphoryl benzynes via a nucleophilic attack. Thus, this report illustrated novel phosphorus-functionalized benzynes and their regioselective formation of various poly-substituted organophosphorus arenes.


 Please wait while we load your content...
Please wait while we load your content...