Synthesis and reactivity of the apically functionalized (pseudo)macrobicyclic iron(ii) tris-dioximates and their hybrid phthalocyaninatoclathrochelate derivatives comprising reactive and vector terminal groups†
Abstract
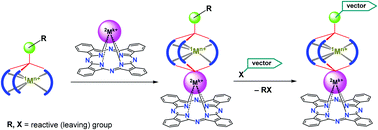
Binuclear FeII, ZrIV(HfIV)-containing phthalocyaninatoclathrochelates with a terminal reactive formyl group were prepared using three synthetic approaches, (i) the direct template condensation of appropriate chelating and capping ligand synthons on the iron(II) ion as a matrix, (ii) a transmetallation of the initially obtained macrobicyclic precursors with labile capping fragment(s), and (iii) a macrobicyclization of the suitable semiclathrochelate intermediates. The latter pathway was found to be the most favorable synthetic approach, typically giving the target hybrid compound in its highest yield. The single terminal formyl group of the thus obtained iron(II) phthalocyaninatoclathrochelates was functionalized using a post-synthetic strategy via a hydrazonate condensation with isoniazid, giving the corresponding cage complex with a biorelevant pharmacophoric fragment. These binuclear compounds and their semiclathrochelate precursors were characterized using elemental analysis, MALDI-TOF mass spectrometry, UV-vis, 1H and 13C {1H} NMR spectroscopirs, and the single crystal X-ray diffraction experiments. The ditopic hybrid molecules contain two relatively independent chromophoric centers (i.e., a metallo(IV)phthalocyaninate core and a semiclathrochelate iron(II)-centered tris-α-dioximate fragment). As follows from XRD data, the geometry of their FeN6-polyhedra is close to intermediate between a trigonal prism (the distortion angle φ = 0°) and a trigonal antiprism (φ = 60°). The capping heptacoordinate metal(IV) ions form their MIVN4O3-coordination polyhedra and are shifted from the mean plane of a phthalocyanine macrocycle at a distance from 0.9 to 1.2 Å. The developed multistep synthetic approach is proposed as a general convenient and straightforward strategy for obtaining a wide range of binuclear hybrid cage complexes of this type, functionalized for their target delivery to various biosystems (biotargets).


 Please wait while we load your content...
Please wait while we load your content...