Ditriphenylenothiophene butterfly-shape liquid crystals. The influence of polyarene core topology on self-organization, fluorescence and photoconductivity†
Abstract
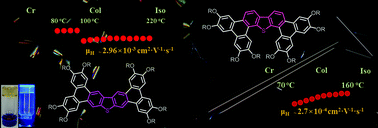
Two series of regio-isomeric butterfly-shaped liquid crystalline systems, based on a ditriphenylenothiophene core (α/β-DTPT) equipped with eight peripheral long alkoxy chains, were synthesized in two steps by the Suzuki–Miyaura cross-coupling/Scholl cyclo-dehydrogenation reactions tandem from appropriate precursors. The influence of the core topology (i.e. positional isomerism) on the mesomorphism, gelling self-assembly, absorption and luminescence, and charge-transport properties of both sets of isomers was investigated and compared. The six fused π-conjugated molecules systematically display columnar hexagonal (Colhex) mesophases with β-fused ditriphenylenothiophene compounds (DTPTBn) showing more extended mesophase ranges and higher phase transition temperatures than their isomeric α-counterparts (DTPTAn). All ditriphenylenothiophenes also show strong aggregation behavior in organic solvents, and, in addition, good organic gelling abilities were found for the β-isomers. Interestingly too, high hole mobility rates greater than 10−3 cm2 V−1 s−1 were measured for the β-isomers, which were about 10 times higher than those of the α-counterparts. Modeling of the molecular structures combined with DFT calculations showed that the β-isomers possess flatter and more extended π-conjugated cores than the slightly distorted α-isomers, and consequently were more prone to optimize π–π intermolecular interactions, in agreement with the high-temperature mesophases and high charge carrier mobilities observed for these isomers. Finally, both sets of compounds emit blue-purple light in solution and the emission is substantially red-shifted when organized in films. DFT calculations of the HOMO–LUMO energy levels were also consistent with the optical measurements. This original molecular design and straightforward synthesis combination provides an efficient way to obtain novel π-extended organic, potentially semiconductor materials for further electronic device investigations.


 Please wait while we load your content...
Please wait while we load your content...