Structural and ethylene oligomerization studies of chelating (imino)phenol Fe(ii), Co(ii) and Ni(ii) complexes: an experimental and theoretical approach†
Abstract
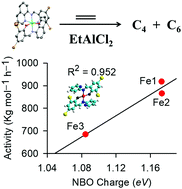
The metal complexes [Fe(L1)2] (Fe1); [Fe(L2)2] (Fe2); [Fe(L3)2] (Fe3); [Co(L1)2] (Co1); [Co(L2)2] (Co2); [Co(L3)3] (Co3); [Ni(L1)2] (Ni1); [Ni(L2)2] (Ni2) and [Ni(L3)3] (Ni3); where L = 2,4-dibromo-6-((pyridin-2-ylimino)methyl)phenol (L1H), 2,4-dibromo-6-(((4-methylpyridin-2-yl)imino)methyl)phenol (L2H) and 2,4-dibromo-6-((quinolin-8-ylimino)methyl)phenol (L3H), were synthesized in good yields. The complexes were characterized using IR spectroscopy, UV-visible spectroscopy, mass spectrometry, magnetic moment measurements, elemental analysis, and X-ray crystallography. The molecular structures of complexes Fe3a (oxidised form of Fe3) and Ni3 confirmed the isolation of bis(chelated) tridentate bound octahedral compounds. Activation of the complexes with the EtAlCl2 co-catalyst produced active catalysts in the ethylene oligomerization reactions to afford mainly C4 and C6 oligomers. The catalytic activities and product distribution were largely controlled by the nature of the ligand and the metal atom. Density functional theory calculations were used to investigate the influence of complex properties and global descriptors in the ethylene oligomerization reactions. The stability and magnitude of the charge of the metal atom appear to drive the overall catalytic activities of the complexes.


 Please wait while we load your content...
Please wait while we load your content...