Metathesis as an alternative synthesis route to layered sulfides A(LiZn)S2 (A = alkali-metal) with unexpected colors
Abstract
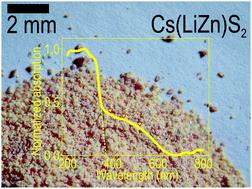
Pure powders of Na(LiZn)S2 can be obtained through a solid-state reaction, and A(LiZn)S2 (A = K, Rb, Cs) result from metathesis reactions between alkali-metal chlorides and the same constituents used to prepare the Na(LiZn)S2 powder. Hence, the metathesis reaction enables extended sulphide chemistry without the use of either H2S gas or very reactive starting materials. By the metathesis reaction it was possible to obtain relatively pure Cs(LiZn)S2. Trigonal Na(LiZn)S2 and tetragonal A(LiZn)S2 (A = K, Rb, Cs) exhibit significant structural similarities, having (LiZn)S2 layers that are separated by alkali-metals (Na–Cs). Against expectations, Cs(LiZn)S2 is orange red in colour, Rb(LiZn)S2 is strongly yellow, K(LiZn)S2 is pale yellow, and Na(LiZn)S2 is colourless. Ultraviolet-visible spectroscopy data on Cs(LiZn)S2 and Na(LiZn)S2 contain several shoulders apart from apparent band-edges close to 3.3 eV. In the former, it seems as if the optical excitations range all the way into green (∼600 nm), which concurs with the observed red colour. Nuclear magnetic resonance investigations on cores 133Cs, 23Na, and 7Li suggest that these ions are firmly held in the atomic lattice, as judged by the resonance frequency widths and relatively long nuclear spin relaxation times (T1), ranging from 10 to 200 seconds for 23Na and 7Li. So there should be only electronic excitations in these compounds. Band-structure calculations of Li–Zn ordered versions of the lattices suggest a direct band-gap in both compounds, corresponding to an excitation from sulphur to zinc. The theoretical band-gaps amount to 2.54 eV for CsLiZnS2 and 1.85 eV for NaLiZnS2, and the steep edges in the density of states are found at 3.3 eV for both cases. As no Li–Zn ordering is observed by X-ray diffraction, there must be an inherent atomic disorder. By theoretical simulations, local Li–Zn anti-site orderings were introduced and the resulting electronic structure was evaluated. However, the simulated optical behaviour could only tentatively explain the spectroscopic data of Na(LiZn)S2; the orange red colour of Cs(LiZn)S2 must be an even more complex phenomenon, as the Li–Zn simulations were insufficient to explain the relatively strong optical activity in the range between 400 and 600 nm.


 Please wait while we load your content...
Please wait while we load your content...