N-Methylamide-structured SB366791 derivatives with high TRPV1 antagonistic activity: toward PET radiotracers to visualize TRPV1†
Abstract
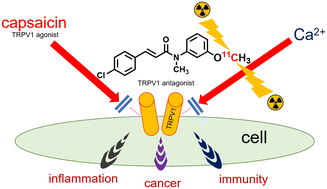
Transient receptor potential cation channel subfamily V member 1 (TRPV1)-targeted compounds were synthesized by modifying the structure of SB366791, a pharmaceutically representative TRPV1 antagonist. To avoid amide–iminol tautomerization, structurally supported N-methylated amides (i.e., 3-alkoxy-substitued N-meythylamide derivatives of SB366791) were evaluated using a Ca2+ influx assay, in which cells expressed recombinant TRPV1 in the presence of 1.0 μM capsaicin. The antagonistic activities of N-(3-methoxyphenyl)-N-methyl-4-chlorocinnamamide (2) (RLC-TV1004) and N-{3-(3-fluoropropoxy)phenyl}-N-methyl-4-chlorocinnamamide (4) (RLC-TV1006) were found to be approximately three-fold higher (IC50: 1.3 μM and 1.1 μM, respectively) than that of SB366791 (IC50: 3.7 μM). These results will help reinvigorate the potential of SB366791 in medicinal chemistry applications. The 3-methoxy and 3-fluoroalkoxy substituents were used to obtain radioactive [11C]methoxy- or [18F]fluoroalkoxy-incorporated tracers for in vivo positron emission tomography (PET). Using the 11C- or 18F-labeled derivatives, explorative PET imaging trials were performed in rats.


 Please wait while we load your content...
Please wait while we load your content...