Emission colours of bistable photochromic compounds: Ce-doped alkaline (Rb, K, Na)–indium fluorides†
Abstract
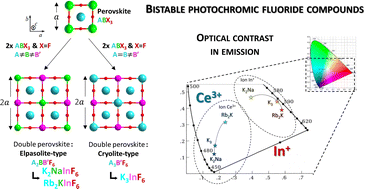
The structural and spectroscopic properties of three Ce3+-doped A3InF6 compounds (with A+ = Rb+, K+, Na+) are investigated. Containing indium, they all exhibit 2Ce3+ + In3+ ⇔ In+ + 2Ce4+ redox processes induced by UV irradiation between Ce3+ and In3+ cations. The photochromo-luminescent behaviour and associated kinetics, with the Ce3+ and In+ ion emission quenching through the redox equation, respectively, are investigated. Our studies highlight that different mechanisms (surface vs. bulk) need be considered to explain the decrease in the blue (Ce3+) and orange (In+) emission curves. Moreover, the comparison of Ce3+-doped Rb2KInF6, K2NaInF6 elpasolites and K3InF6 cryolite spectral distributions shows that changing the nature of the alkali cation makes colour tuning possible. The shift of the Ce3+ and In+ colour emissions between the three as-prepared compounds is mainly due to the variation in the ionic-covalent nature of the chemical bonds around the active cation sites. The final calculated optical difference between the two bistable states varies from 0.31 to 0.48 depending on the alkali subnetwork.


 Please wait while we load your content...
Please wait while we load your content...