Differentiating chemical and electrochemical degradation of lithium germanium thiophosphate and the role of atomic layer deposited protection layers†
Abstract
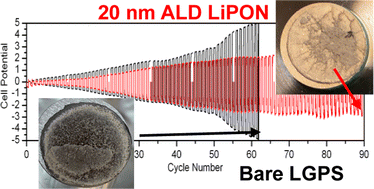
Li10GeP2S12 (LGPS) is a superionic conductor that has an ionic conductivity equivalent to conventional liquid electrolytes (∼10−2 S cm−1) and thus shows exceptional potential to fulfill the promise of solid-state batteries. Nonetheless, LGPS is chemically and electrochemically unstable against Li metal, decomposing into the thermodynamically favorable byproducts of Li3P, Li2S, and alloyed LixGe. Contact between Li metal and LGPS results in formation of high impedance interphase layers due to lithium diffusion into and subsequent reaction with the LGPS structure. Artificial solid electrolyte interphase (ASEI) layers are a promising route to mitigate and reduce the chemical reactivity of the LGPS surface. Here, we differentiate between static chemical degradation induced by LGPS-Li contact, from electrochemical degradation induced via galvanostatic cycling of Li/LGPS/Li cells as critical to rational ASEI evaluation. From this perspective, we utilize a thin ASEI coating of lithium phosphorous oxynitride (LiPON), deposited by atomic layer deposition (ALD), to mitigate both chemical and electrochemical degradation at the Li/LGPS interface.


 Please wait while we load your content...
Please wait while we load your content...