Ambient pressure synthesis of unstable bulk phases of strongly correlated rare-earth nickelates†
Abstract
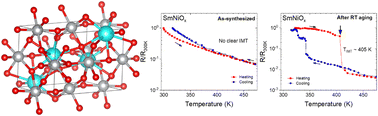
Despite the outstanding electrical, electrochemical, and optical properties of rare-earth (R)-doped nickelates (RNiO3), a bottleneck in its device applications is the need for high-pressure, typically in excess of 100 bars, to stabilize this phase during synthesis. To date, no known near-ambient pressure synthesis process exists for the synthesis of bulk RNiO3 with ionic radii of R lower than that of Nd (such as Sm, Eu, and Gd) in the lanthanide series due to the increasing thermodynamic instability of Ni3+ cations at ambient pressures. In the present study, we report a set of conditions for the successful synthesis of bulk SmNiO3 and NdNiO3 through a sol–gel synthesis procedure followed by annealing at ambient pressure to stabilize Ni3+. Rietveld refinement analysis shows the composition of crystalline SmNiO3 and NdNiO3 phases to be as high as 50 wt% and 96 wt%, respectively. Consequently, sharp, well-defined insulator–metal transitions (IMTs) involving resistance changes of 2–3 orders of magnitude could be achieved, which is comparable to that seen in high-pressure synthesized samples reported in the literature.


 Please wait while we load your content...
Please wait while we load your content...