In situ Fmoc removal – a sustainable solid-phase peptide synthesis approach†
Abstract
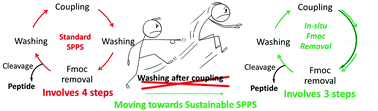
Solid-phase peptide synthesis (SPPS) is the strategy of choice for the synthesis of peptides for research and production purposes. From a green chemistry perspective, SPPS has several positive features. However, it is hampered by high solvent consumption for washings after each of the two main steps, namely deprotection and coupling. Here we propose to combine the two steps into one. In this regard, once the coupling is completed, piperidine or 4-methylpiperidine is added up to a concentration of 20% to the coupling cocktail, which contains an excess of Fmoc-aa-OxymaPure (active ester) and Fmoc-peptide resin. We further demonstrate that the deactivation of the OxymaPure ester is faster than Fmoc removal and therefore this in situ Fmoc removal strategy avoids the double incorporation of the amino acid into the peptide chain. Furthermore, we also show that this single wash is more efficient at removing traces of piperidine when 1% of OxymaPure, which is a weak acid, is added to the washing solvent. This strategy brings about a saving of 75% of the solvent. We envisage that the modifications to this new protocol will be added to the green toolbox for SPPS and will make this strategy more sustainable.


 Please wait while we load your content...
Please wait while we load your content...