Curcumin alleviates LPS-induced intestinal homeostatic imbalance through reshaping gut microbiota structure and regulating group 3 innate lymphoid cells in chickens†
Abstract
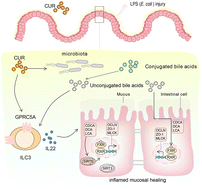
Gastrointestinal dysfunction is associated with a disturbance of immune homeostasis, changes in the intestinal microbiome, alteration of the composition of the bile acid pool, and dynamic imbalance of group 3 innate lymphoid cells (ILC3s). Curcumin (CUR), a polyphenolic compound isolated from turmeric, has been known to attenuate intestinal inflammation in potential therapies for gastrointestinal diseases. It was hypothesized that CUR could target the gut microbiome to modulate bile acid (BA) metabolism and the function of ILC3s in ameliorating lipopolysaccharide (LPS)-induced imbalance of intestinal homeostasis in chickens. Seven hundred and twenty 1-day-old crossbred chickens were randomly divided into four treatments, namely CON_saline (basal diet + saline control), CUR_saline (basal diet + 300 mg kg−1 curcumin + saline), CON_LPS (basal diet + LPS), and CUR_LPS (basal diet + 300 mg kg−1 curcumin + LPS), each consisting of 6 replicates of 30 birds. On days 14, 17, and 21, the chickens in the CON_LPS and CUR_LPS treatments were intraperitoneally injected with LPS at 0.5 mg per kg BW. Dietary CUR supplementation significantly decreased LPS-induced suppression of growth performance and injury to the intestinal tight junctions and decreased the vulnerability to LPS-induced acute inflammatory response by inhibiting pro-inflammatory (interleukin-1β and tumor necrosis factor-α) cytokines. CUR reshaped the cecal microbial community and BA metabolism, contributing to regulation of the intestinal mucosal immunity by promoting the anti-inflammatory (interleukin 10, IL-10) cytokines and enhancing the concentrations of primary and secondary BA metabolites (chenodexycholic acid, lithocholic acid). LPS decreased farnesoid X receptor (FXR) and G protein-coupled receptor class C group 5 member A synthesis, which was reversed by CUR administration, along with an increase in interleukin 22 (IL-22) production from ILC3s. Dietary supplementation of CUR increased the prevalence of Butyricicoccus and Enterococcus and enhanced the tricarboxylic acid cycle of intestinal epithelial cells. In addition, curcumin supplementation significantly increased sirtuin 1 and sirtuin 5 transcription and protein expression, which contributes to regulating mitochondrial metabolic and oxidative stress responses to alleviate LPS-induced enteritis. Our findings demonstrated that curcumin played a pivotal role in regulating the structure of the intestinal microbiome for health promotion and the treatment of intestinal dysbiosis. The beneficial effects of CUR may be attributed to the modulation of the BA-FXR pathway and inhibition of inflammation that induces IL-22 secretion by ILC3s in the intestinal lamina propria.

 Please wait while we load your content...
Please wait while we load your content...