Effects of decaffeinated green coffee extract supplementation on anthropometric indices, blood glucose, leptin, adiponectin and neuropeptide Y (NPY) in breast cancer survivors: a randomized clinical trial
Abstract
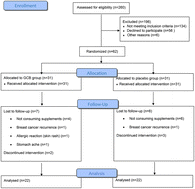
Objective: This study aimed to evaluate the effects of decaffeinated green coffee extract (DGCE) supplementation on anthropometric indices, blood glucose, leptin, adiponectin, and neuropeptide Y (NPY) in breast cancer survivors with obesity. Method: A total of 44 breast cancer survivors with obesity aged between 18 and 70 years and with a mean body mass index (BMI) of 31.62 ± 4.97 kg m−2 participated in this double-blind randomized clinical trial. Eligible patients were randomized to the intervention (n = 22) and control (n = 22) groups. They received two 400 mg capsules of DGCE or two identical placebos daily for 12 weeks. Serum concentrations of leptin, adiponectin, NPY, fasting blood sugar, insulin, and homeostatic model assessment for insulin resistance (HOMA-IR) were measured at the baseline and after completion of the intervention. Also, weight, waist circumference, fat percentage, muscle percentage, and visceral fat were measured. Results: There were no significant differences in terms of changes of anthropometric indices and concentrations of leptin, adiponectin, NPY, and blood sugar between the two studied groups. Conclusion: Supplementation with DGCE in breast cancer survivors with obesity had no significant effect on anthropometric indices and blood glucose, leptin, adiponectin, and NPY levels.


 Please wait while we load your content...
Please wait while we load your content...