Direct inhibition of the first PDZ domain of ZO-1 by glycyrrhizin is a possible mechanism of tight junction opening of Caco-2 cells†
Abstract
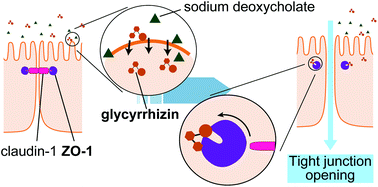
Glycyrrhizin (GL) is known to exhibit a variety of useful pharmacological activities, including anti-inflammation, anti-hepatotoxicity, and enhancement of intestinal drug absorption. GL has been reported to modify the assembly of actin filaments, thereby modulating tight junction (TJ) integrity, but the detailed molecular mechanisms of this remain unclear. In this study, we first found that GL binds to the first PDZ domain of zonula occludens-1 (ZO-1(PDZ1)) through NMR experiments. The structure of the GL–ZO-1(PDZ1) complex was then constructed using HADDOCK with the transferred nuclear Overhauser effect-based inter-hydrogen distance constraints as well as restrictions on the interfacial residues identified from 1H–15N HSQC spectral changes. We identified the relevant interactions between the glucuronate-2 moiety of GL and the carboxylate binding loop of the ligand binding site of ZO-1(PDZ1). We further examined the interaction of ZO-1(PDZ1) with glycyrrhetinic acid and with GA-3-monoglucuronide and observed a much lower affinity for each than for that with GL, with good agreement with the model. The other contacts found in the model were examined by using an amino acid substitution mutant of ZO-1(PDZ1). Finally, we reproduced the experiments reported by Sakai et al. in which high-dose GL prolonged the TJ-opening mediated with sodium deoxycholate as indicated by reduced transepithelial electrical resistance.


 Please wait while we load your content...
Please wait while we load your content...