Anisotropic oxidative growth of goethite-coated sand particles in column reactors during 4-chloronitrobenzene reduction by Fe(ii)/goethite†
Abstract
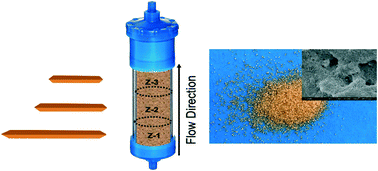
Reduction of nitroaromatic compounds (NACs), an important class of groundwater pollutants, by Fe(II) associated with iron oxides, a highly reactive reductant in anoxic aquifers, has been studied widely, but there are significant differences between the well-controlled, batch reactor conditions of the laboratory and the complicated conditions encountered in the field. Continuous flow column reactors containing goethite-coated sand and aqueous carbonate buffer were continuously exposed to 0.05 mM 4-chloronitrobenzene (4-ClNB) and 0.5 mM Fe(II) to emulate more realistic scenarios and to allow study of the oxidative growth of goethite particles using both saturated and unsaturated flow conditions. The experiments were designed to test how attachment to a surface affected particle growth and how particle growth affected the extent of reaction over time. After reaction, particles from different sections of each column were collected, and the goethite was detached from the sand grains for characterization using transmission electron microscopy. The amount of oxidative growth varied as a function of distance from the column inlet, with the most growth observed at the inlet end (bottom) of the column. Similar to previous work using batch reactors, newly oxidized Fe(III) was mostly added to the goethite particle tips, resulting in up to an 81% increase in length under saturated flow and a 50% increase in length under unsaturated flow after 220 pore volumes. With saturated flow, reactant concentrations and the extent of the reaction are important factors determining the extent of mineral growth. For unsaturated column conditions, however, flow path substantially impacts mineral growth in the column. Reactors sacrificed after 220 pore volumes under saturated flow conditions resulted in an overall 70% increase in goethite mass while the unsaturated flow column resulted in a 40% increase in goethite mass, more variable mineral growth as a function of distance from the inlet, and overall, 50% less 4-ClNB conversion. The results demonstrate that quantitative characterization of oxidative mineral growth of goethite nanoparticles attached to an underlying mineral is practical and elucidates the major variables impacting the reactivity of mineral nanoparticles in contaminated groundwater systems.
- This article is part of the themed collection: Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...