Crystal orientation-dependent etching and trapping in thermally-oxidised Cu2O photocathodes for water splitting†
Abstract
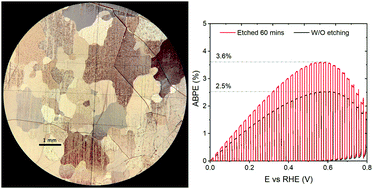
Ammonia solution etching was carried out on thermally-oxidised cuprous oxide (TO-Cu2O) in photocathode devices for water splitting. The etched devices showed increased photoelectrochemical (PEC) performance compared to the unetched ones as well as improved reproducibility. −8.6 mA cm−2 and −7 mA cm−2 photocurrent density were achieved at 0 V and 0.5 V versus the reversible hydrogen electrode (VRHE), respectively, in the champion sample with an onset potential of 0.92 VRHE and a fill factor of 44%. An applied bias photon-to-current efficiency of 3.6% at 0.56 VRHE was obtained, which represents a new record for Cu2O-based photocathode systems. Capacitance-based profiling studies showed a strong pinning effect from interfacial traps in the as-grown device, and these traps were removed by ammonia solution etching. Moreover, the etching procedure gave rise to a diverse morphology of Cu2O crystals based on the different crystallographic orientations. The distribution of crystallographic orientations and the relationship between the crystal orientation and the morphology after etching were examined by electron backscatter diffraction (EBSD) and scanning electron microscopy (SEM). The high-index crystal group showed a statistically higher PEC performance than the low-index group. X-ray photoelectron spectroscopy (XPS) and transmission electron microscopy (TEM) revealed metallic copper at the Cu2O/Ga2O3 interface, which we attribute as the dominant trap that limits the PEC performance. It is concluded that the metallic copper originates from the reduction of the CuO impurity layer on the as-grown Cu2O sample during the ALD process, while the reduction from Cu2O to Cu is not favourable.
- This article is part of the themed collection: The EES Family Board Members Collection


 Please wait while we load your content...
Please wait while we load your content...