A synthetic tactic to substitute axial ligands in sterically demanding Ru(ii)porphyrinates†
Abstract
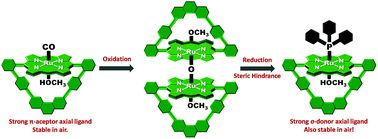
We report a synthetic strategy that allows for the preparation of sterically encumbered heteroleptic Ru(II)porphyrinates with the desired configuration of stable/inert and weak/labile axial ligands to direct reactions between substrates to exclusively occur at the sterically encumbered face. To demonstrate the method, we describe the synthesis of a strapped-Ru(II)porphyrinate bearing a stable/inert triphenylphosphine (PPh3) bulky axial ligand coordinated exo to the central cavity and a weak/labile methanol molecule coordinated at the internal axial position. With this axial ligand configuration, the reported Ru(II)porphyrinate exclusively promotes carbene transfer reactions to olefins through the central cavity, which has been verified by the selective formation of cycloprane-linked [2]rotaxanes.


 Please wait while we load your content...
Please wait while we load your content...