Water soluble organometallic small molecules as promising antibacterial agents: synthesis, physical–chemical properties and biological evaluation to tackle bacterial infections†
Abstract
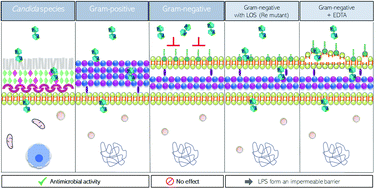
The Na[3,3′-Fe(8-I-1,2-C2B9H10)2] and Na[2,2′-M(1,7-C2B9H11)] (M = Co3+, Fe3+) small molecules are synthesized and the X-ray structures of [(H3O)(H2O)5][2,2′-Co(1,7-C2B9H11)2] and [Cs(MeCN)][8,8′-I2-Fe(1,2 C2B9H10)2], both displaying a transoid conformation of the [M(C2B9)2]− framework, are reported. Importantly, the supramolecular structure of [(H3O)(H2O)5][2,2′-Co(1,7-C2B9H11)2] presents 2D layers leading to a lamellar arrangement of the anions while the cation layers form polymeric water rings made of six- and four-membered rings of water molecules connected via OH⋯H hydrogen bonds; B–H⋯O contacts connect the cationic and anionic layers. Herein, we highlight the influence of the ligand isomers (ortho-/meta-), the metal effect (Co3+/Fe3+) on the same isomer, as well as the influence of the presence of the iodine atoms on the physical–chemical and biological properties of these molecules as antimicrobial agents to tackle antibiotic-resistant bacteria, which were tested with four Gram-positive bacteria, five Gram-negative bacteria, and three Candida albicans strains that have been responsible for human infections. We have demonstrated an antimicrobial effect against Candida species (MIC of 2 and 3 nM for Na[3,3′-Co(8-I-1,2-C2B9H10)2] and Na[2,2′-Co(1,7-C2B9H11)2], respectively), and against Gram-positive and Gram-negative bacteria, including multiresistant MRSA strains (MIC of 6 nM for Na[3,3′-Co(8-I-1,2-C2B9H10)2]). The selectivity index for antimicrobial activity of Na[3,3′-Co(1,2-C2B9H11)2] and Na[3,3′-Co(8-I-1,2-C2B9H10)2] compounds is very high (165 and 1180, respectively), which reveals that these small anionic metallacarborane molecules may be useful to tackle antibiotic-resistant bacteria. Moreover, we have demonstrated that the outer membrane of Gram-negative bacteria constitutes an impermeable barrier for the majority of these compounds. Nonetheless, the addition of two iodine groups in the structure of the parent Na[3,3′-Co(1,2-C2B9H11)2] had an improved effect (3–7 times) against Gram-negative bacteria. Possibly the changes in their physical–chemical properties make the meta-isomers and the ortho-di-iodinated small molecules more permeable for crossing this barrier. It should be emphasized that the most active metallabis(dicarbollide) small molecules are both transoid conformers in contrast to the ortho- [3,3′-Co(1,2-C2B9H11)2]− that is cisoid. The fact that these small molecules cross the mammalian membrane and have antimicrobial properties but low toxicity for mammalian cells (high selectivity index, SI) represents a promising tool to treat infectious intracellular bacteria. Since there is an urgent need for antibiotic discovery and development, this study represents a relevant advance in the field.


 Please wait while we load your content...
Please wait while we load your content...