Geminal C–Cl and Si–Cl bond activation of chloromethanes and chlorosilanes by gallanediyl LGa†
Abstract
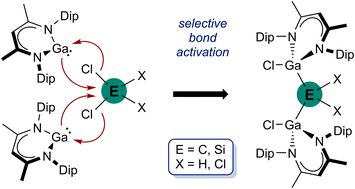
The activation of relatively inert E–X σ-bonds by low-valent main group metal complexes is receiving increasing interest. We here confirm the promising potential of gallanediyl LGa (L = HC[C(Me)N(Dip)]2, Dip = 2,6-i-Pr2C6H3) to activate E–Cl (E = C, Si) σ-bonds of group 14 element compounds. Equimolar reactions of LGa with chloromethanes and chlorosilanes EHxCl4−x (E = C, x = 0–2; E = Si, x = 0, 1) occurred with E–Cl bond insertion and formation of gallylmethanes and -silanes L(Cl)GaEHxCl3−x (E = C, x = 2 (1), 1 (2), 0 (3); E = Si, x = 1 (4)). In contrast, consecutive insertion into a geminal E–Cl bond was observed with two equivalents of LGa, yielding digallyl complexes [L(Cl)Ga]2EHxCl2−x (E = C, x = 2 (5); E = Si, x = 1 (6), 0 (7)). Compounds 1–7 were characterized by heteronuclear NMR (1H, 13C, 29Si (4, 6)), IR spectroscopy and elemental analysis, and their solid-state structures were determined by single-crystal X-ray diffraction (sc-XRD).


 Please wait while we load your content...
Please wait while we load your content...