Direct decarboxylative Giese reactions
Abstract
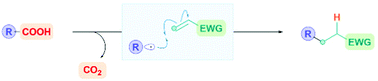
The quest to find milder and more sustainable methods to generate highly reactive, carbon-centred intermediates has led to a resurgence of interest in radical chemistry. In particular, carboxylic acids are seen as attractive radical precursors due their availability, low cost, diversity, and sustainability. Moreover, the corresponding nucleophilic carbon-radical can be easily accessed through a favourable radical decarboxylation process, extruding CO2 as a traceless by-product. This review summarizes the recent progress on using carboxylic acids directly as convenient radical precursors for the formation of carbon–carbon bonds via the 1,4-radical conjugate addition (Giese) reaction.


 Please wait while we load your content...
Please wait while we load your content...