Spectroscopic investigation of photophysics and tautomerism of amino- and nitroporphycenes†
Abstract
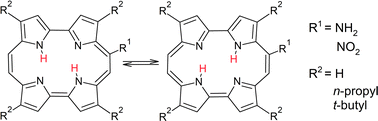
Parent, unsubstituted porphycene and its two derivatives: 2,7,12,17-tetra-n-propylporphycene and 2,7,12,17-tetra-t-butylporphycene were substituted at the meso position with amino and nitro groups. These two families of porphycenes were characterized in detail with respect to their spectral, photophysical, and tautomeric properties. Two trans tautomers of similar energies coexist in the ground electronic state, but only one form dominates in the lowest excited singlet state. Absorption, magnetic circular dichroism (MCD), and emission anisotropy combined with quantum-chemical calculations led to the assignment of S1 and S2 transitions in both tautomers. Compared with the parent porphycene, the S1–S2 energy gap significantly increases; for one tautomeric form, the effect is twice as large as for the other. Both amino- and nitroporphycenes emit single fluorescence; previously reported dual emission of aminoporphycenes is attributed to a degradation product. Introduction of bulky t-butyl groups leads to a huge decrease in fluorescence intensity; this effect, arising from the interaction of the meso substituent with the adjacent t-butyl moiety, is particularly strong in the nitro derivative.


 Please wait while we load your content...
Please wait while we load your content...