Using diketopyrrolopyrroles to stabilize double excitation and control internal conversion†
Abstract
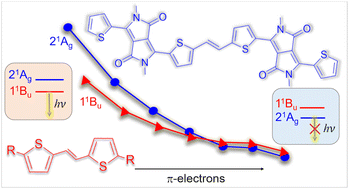
Diketopyrrolopyrrole (DPP) is a pivotal functional group to tune the physicochemical properties of novel organic photoelectronic materials. Among multiple uses, DPP–thiophene derivatives forming a dimer through a vinyl linker were recently shown to quench the fluorescence observed in their isolated monomers. Here, we explain this fluorescence quenching using computational chemistry. The DPP–thiophene dimer has a low-lying doubly excited state that is not energetically accessible for the monomer. This state delays the fluorescence allowing internal conversion to occur first. We characterize the doubly excited state wavefunction by systematically changing the derivatives to tune the π-scaffold size and the acceptor and donor characters. The origin of this state's stabilization is related to the increase in the π-system and not to the charge-transfer features. This analysis delivers core conceptual information on the electronic properties of organic chromophores arranged symmetrically around a vinyl linker, opening new ways to control the balance between luminescence and internal conversion.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...