Two- and three-body fragmentation of multiply charged tribromomethane by ultrafast laser pulses†
Abstract
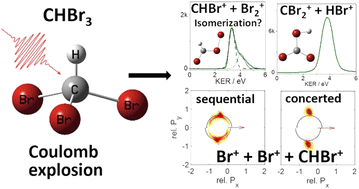
We investigate the two- and three-body fragmentation of tribromomethane (bromoform, CHBr3) resulting from multiple ionization by 28-femtosecond near-infrared laser pulses with a peak intensity of 6 × 1014 W cm−2. The analysis focuses on channels consisting exclusively of ionic fragments, which are measured by coincidence momentum imaging. The dominant two-body fragmentation channel is found to be Br+ + CHBr2+. Weaker HBr+ + CBr2+, CHBr+ + Br2+, CHBr2+ + Br2+, and Br+ + CHBr22+ channels, some of which require bond rearrangement prior to or during the fragmentation, are also observed. The dominant three-body fragmentation channel is found to be Br+ + Br+ + CHBr+. This channel includes both concerted and sequential fragmentation pathways, which we identify using the native frames analysis method. We compare the measured kinetic energy release and momentum correlations with the results of classical Coulomb explosion simulations and discuss the possible isomerization of CHBr3 to BrCHBr–Br (iso-CHBr3) prior to the fragmentation.
- This article is part of the themed collection: Ions, electrons, coincidences and dynamics: Festschrift for John H.D. Eland


 Please wait while we load your content...
Please wait while we load your content...
