Oxygen deficiency in Sr2FeO4−x: electrochemical control and impact on magnetic properties†
Abstract
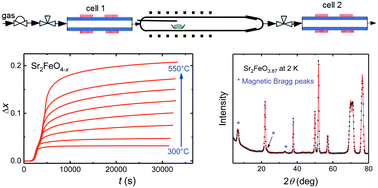
The oxygen-deficient system Sr2FeO4−x was explored by heating the stoichiometric Fe4+ oxide Sr2FeO4 in well-defined oxygen partial pressures which were controlled electrochemically by solid-state electrolyte coulometry. Samples with x up to about 0.2 were obtained by this route. X-ray diffraction analysis reveals that the K2NiF4-type crystal structure (space group I4/mmm) of the parent compound is retained. The lattice parameter a slightly decreases while the c-parameter increases with increasing x, which is in contrast to the Ruddlesden–Popper system Sr3Fe2O7−x and suggests removal of oxygen atoms from FeO2 lattice planes. The magnetic properties were studied by magnetization, 57Fe Mössbauer, and powder neutron diffraction experiments. The results suggest that extraction of oxygen atoms from the lattice progressively changes the elliptical spiral spin ordering of the parent compound to an inhomogeneous magnetic state with coexistence of long-range ordered regions adopting a circular spin spiral and smaller magnetic clusters.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...