Reaction-intermediate-induced atomic mobility in heterogeneous metal catalysts for electrochemical reduction of CO2†
Abstract
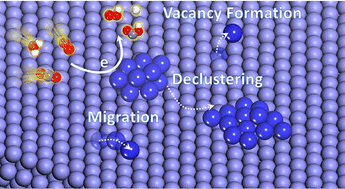
Improving the activity and selectivity of heterogeneous metal electrocatalysts has been the primary focus of CO2 electroreduction studies, however, the stability of these materials crucial for practical application remains less understood. In our work, the impact of the reaction intermediates (RIs) on the energetics and mechanism of metal-atom migration is studied with a combination of density functional theory (DFT) and ab initio molecular dynamics (AIMD) on pure transition metals Cu, Ag, Au, Pd, as well as three Cu4−xPdx (x = 1,2, and 3) alloys. Reaction intermediates (RIs) for the CO2 reduction reaction, H2 evolution, and O2 reduction were considered. The effect of adsorbed RIs was observed to facilitate metal atom migration generally by decreasing the kinetic barriers for migration. The atomic mobility trends in the commonly used CO2RR metal electrocatalysts in the course of electrolysis conditions were established. This study provides theoretical insight into understanding how the electrocatalyst may undergo promoted restructuring in the presence of RIs under realistic electrochemical conditions.


 Please wait while we load your content...
Please wait while we load your content...