Modelling mercury sorption of a polysulfide coating made from sulfur and limonene†
Abstract
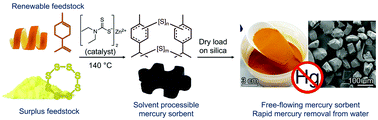
A polymer made from sulfur and limonene was used to coat silica gel and then evaluated as a mercury sorbent. A kinetic model of mercury uptake was established for a range of pH values and concentrations of sodium chloride. Mercury uptake was generally rapid from pH = 3 to pH = 11. At neutral pH, the sorbent (500 mg with a 10 : 1 ratio of silica to polymer) could remove 90% of mercury within one minute from a 100 mL solution containing 5 ppm HgCl2 and 99% over 5 minutes. It was found that sodium chloride, at concentrations comparable to seawater, dramatically reduced mercury uptake rates and capacity. It was also found that the spent sorbent was stable in acidic and neutral media, but degraded at pH 11 which led to mercury leaching. These results help define the conditions under which the sorbent could be used, which is an important advance for using this material in remediation processes.


 Please wait while we load your content...
Please wait while we load your content...