Effect of alkali metal cations on network rearrangement in polyisoprene ionomers†
Abstract
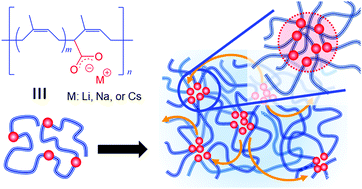
The effects of cations, Li+, Na+, and Cs+, on the structure of ionic aggregates and network rearrangement in carboxylated polyisoprene (PI) ionomers were studied. We found that network rearrangement via interaggregate hopping of metal carboxylates is improved with a decrease in cation size, even though density functional theory (DFT) calculation indicated the increase in the attractive interaction between metal carboxylates. At the same time, we also found that as the size of the cation decreases, the inclusion of the PI segment in the ionic aggregate increases. The DFT calculation suggested the cation–π interaction between the cation and double bonds in the PI segment as the cause for the inclusion. The inclusion of the PI segment with a low glass transition temperature (Tg) plasticizes the ionic aggregate and would sterically hinder the attractive interaction between metal carboxylates. In fact, the electron spin resonance measurement revealed a decrease in the Tg of the ionic aggregate with a decrease in cation size. Based on our findings, we proposed that the inclusion of PI segments in the ionic aggregate is the possible cause for the enhancement of network rearrangement in the carboxylated PI ionomers with a decrease in the cation size.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...