Ethanol electro-oxidation reaction on the Pd(111) surface in alkaline media: insights from quantum and molecular mechanics†
Abstract
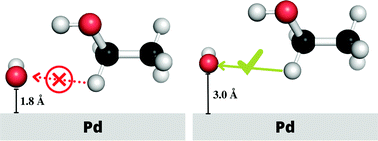
The ethanol electro-oxidation catalyzed by Pd in an alkaline environment involves several intermediate reaction steps promoted by the hydroxyl radical, OH. In this work, we report on the dynamical paths of the first step of this oxidation reaction, namely the hydrogen atom abstraction CH3CH2OH + OH → CH3CHOH + H2O, occurring at the Pd(111) surface and address the thermodynamic stability of the adsorbed reactants by means of quantum and molecular mechanics calculations, with special focus on the effect of the solvent. We have found that the impact of the solvent is significant for both ethanol and OH, contributing to a decrease in their adsorption free energies by a few dozen kcal mol−1 with respect to the adsorption energy under vacuum. Furthermore, we observe that hydrogen atom abstraction is enhanced for those simulation paths featuring large surface–reactant distances, namely, when the reactants weakly interact with the catalyst. The picture emerging from our study is therefore that of a catalyst whose coverage in an aqueous environment is largely dominated by OH with respect to ethanol. Nevertheless, only a small amount of them, specifically those weakly bound to the catalyst, is really active in the ethanol electro-oxidation reaction. These results open the idea of a rational design of co-catalysts based on the tuning of surface chemical properties to eventually enhance exchange current density.


 Please wait while we load your content...
Please wait while we load your content...