The reaction between HgBr and O3: kinetic study and atmospheric implications†
Abstract
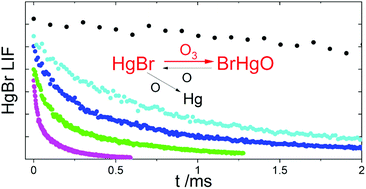
The rate constants of many reactions currently considered to be important in the atmospheric chemistry of mercury remain to be measured in the laboratory. Here we report the first experimental determination of the rate constant of the gas-phase reaction between the HgBr radical and ozone, for which a value at room temperature of k(HgBr + O3) = (7.5 ± 0.6) × 10−11 cm3 molecule s−1 (1σ) has been obtained. The rate constants of two reduction side reactions were concurrently determined: k(HgBr + O) = (5.3 ± 0.4) × 10−11 cm3 molecule s−1 and k(HgBrO + O) = (9.1 ± 0.6) × 10−11 cm3 molecule s−1. The value of k(HgBr + O3) is slightly lower than the collision number, confirming the absence of a significant energy barrier. Considering the abundance of ozone in the troposphere, our experimental rate constant supports recent modelling results suggesting that the main atmospheric fate of HgBr is reaction with ozone to form BrHgO.


 Please wait while we load your content...
Please wait while we load your content...